London Forces Are Also Called

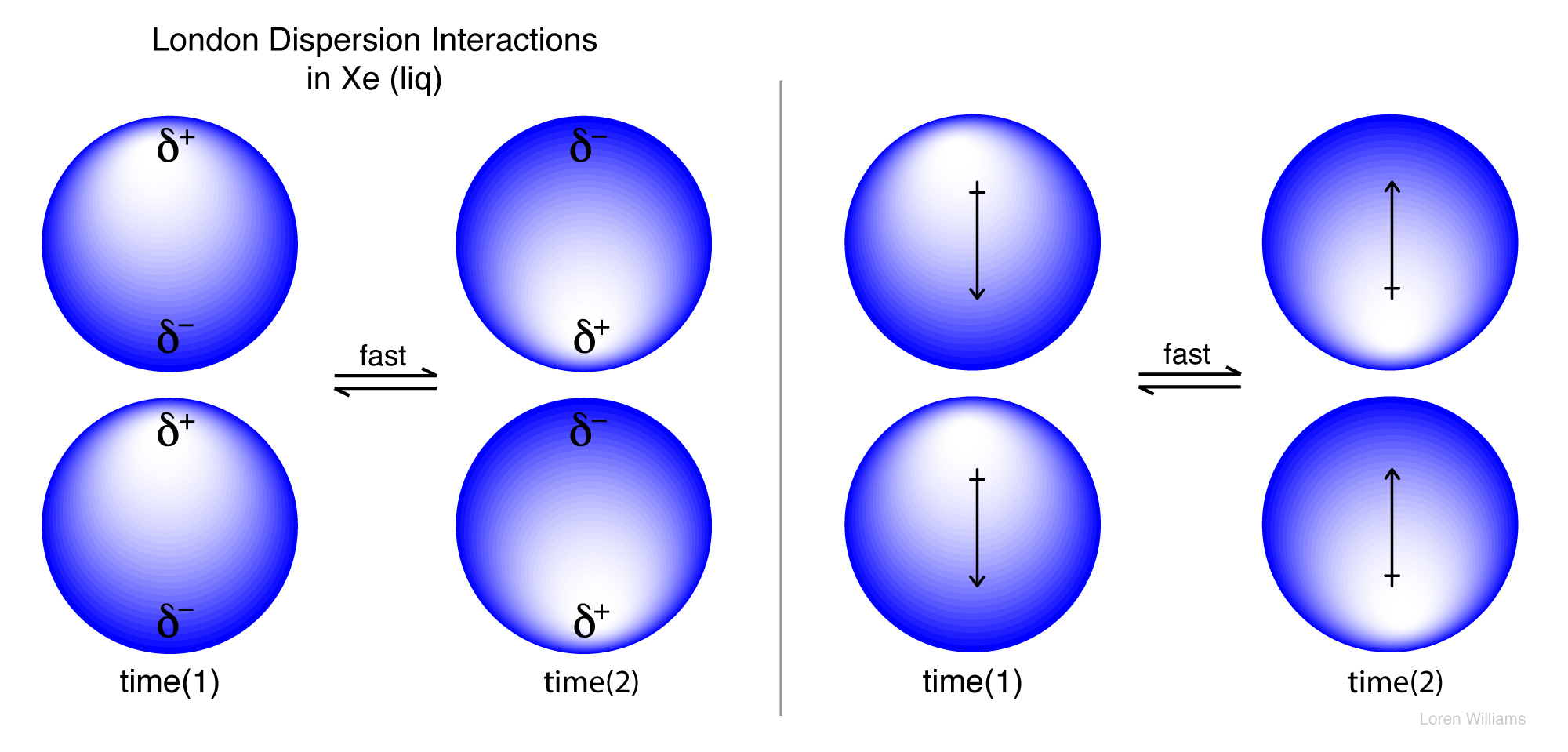
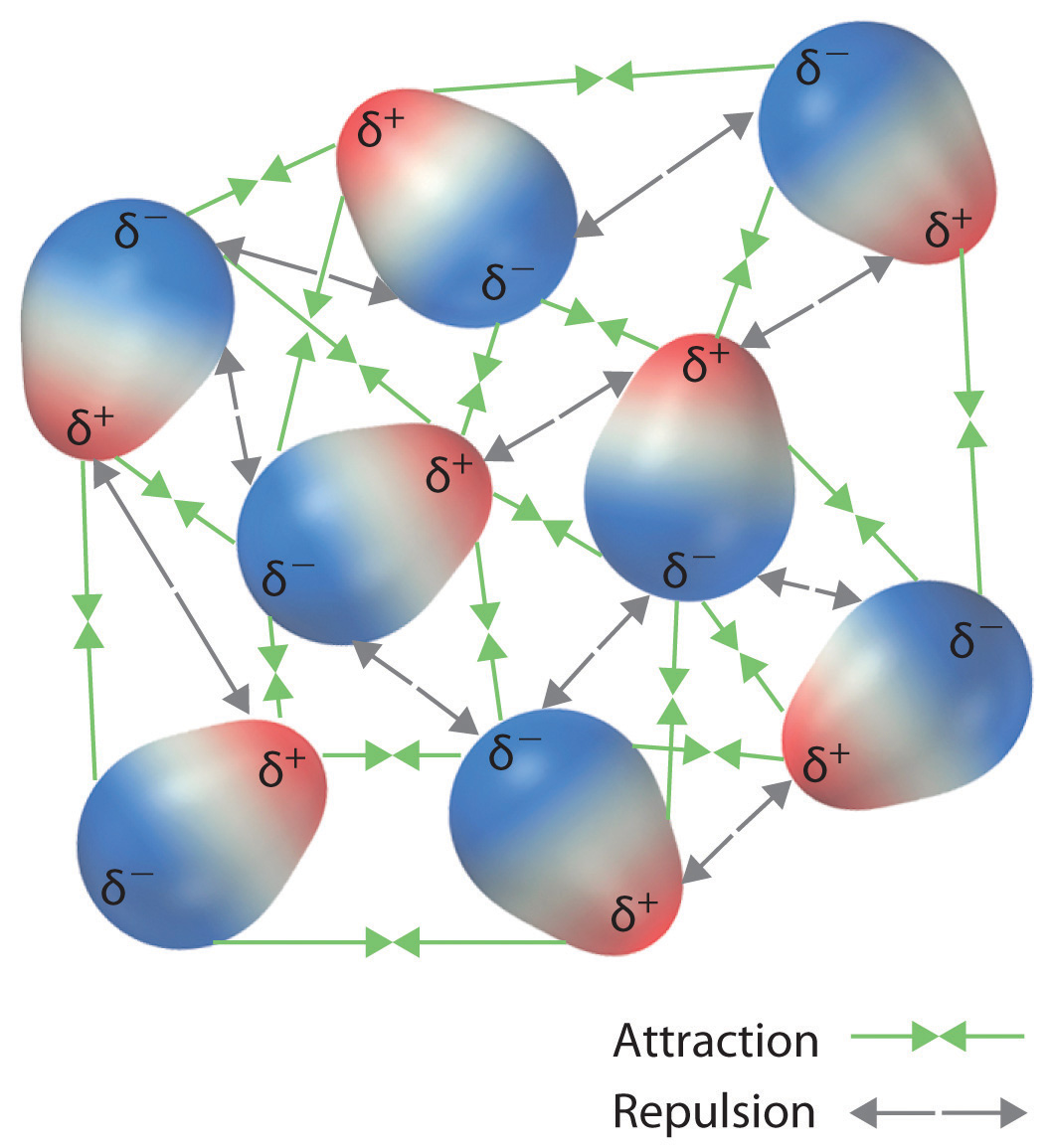
The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.
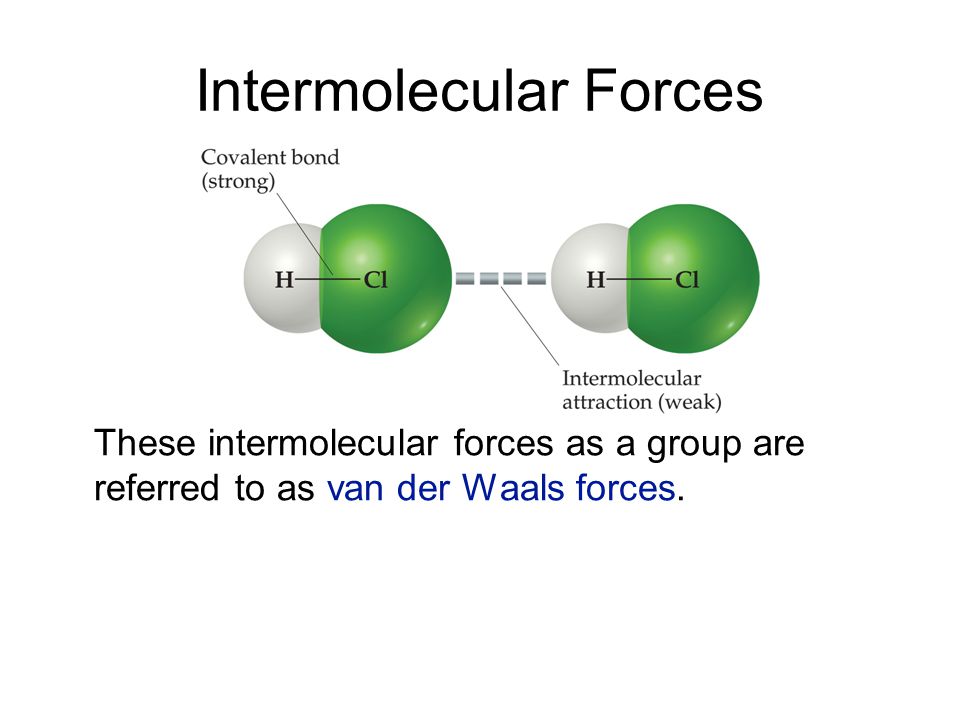
London forces are also called. The ldf is named after the german american physicist fritz london. This force is sometimes called an induced dipole induced dipole attraction. To a first approximation the london force between two molecules is inversely proportional to the seventh power of the distance of separation. They are part of the van der waals forces.
All molecules charged or not polar or not interact by london force s. Dipole dipole interactions by keesom in 1912. All intermolecular attractions are known collectively as van der waals forces. The various different types were first explained by different people at different times.
London dispersion forces are also known as dispersion forces london forces or instantaneous dipole induced dipole forces. It is therefore short range decreasing rapidly as. Dispersion forces for example were described by london in 1930. London dispersion forces ldf also known as dispersion forces london forces instantaneous dipole induced dipole forces or loosely van der waals forces are a type of force acting between atoms and molecules.
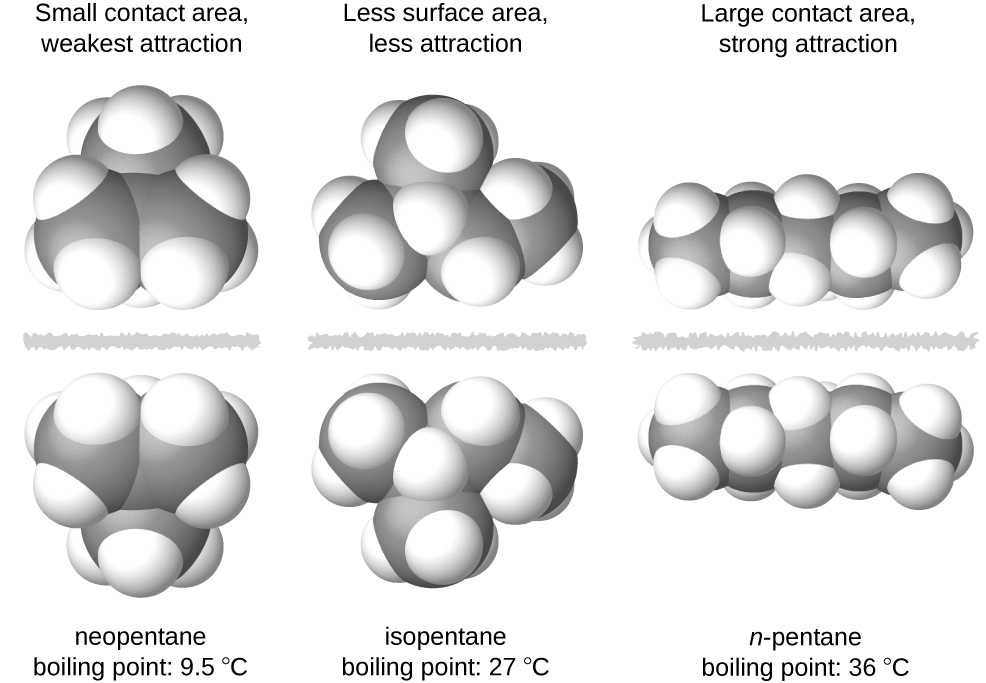
The strength of london dispersion forces is proportional to the polarizability of the molecule which in turn depends on the total number of electrons and the area over which they are spread. Intermolecular forces of attraction called london or dispersion forces. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently.